The UK has become the first country in the world to introduce a dedicated legal framework for the point of care (‘PoC’) and modular manufacture of medicines. The Human Medicines (Amendment) (Modular Manufacture and Point of Care) Regulations 2025 (the ‘Regulations’) seek to give patients access to pioneering treatments whose application has, so far, been potentially hamstrung by fundamental incompatibilities with conventional medicine manufacturing. The landmark legislation is backed by funding, with the UK Government allocating £520 million in grants to the expansion of domestic medicines manufacturing. The focus on manufacturing is one strategy the Government is deploying to realise two key aims of its Life Sciences Sector Plan: to position the UK as the leading life sciences economy in Europe by the end of the decade, and third globally by 2035. With the Regulations now in place, the pharmaceutical sector must adapt quickly to both the opportunities and challenges they present.
Why are the Regulations needed?
Technological innovation has enabled on-demand delivery of everything from media to groceries. In the medical space, the need for speed is much more than a matter of convenience. Increasingly, novel treatments must be delivered quickly regardless of a patient’s location. If not so delivered, medicines with short shelf-lives degrade or patients may become too ill for treatment. The necessary speed and flexibility are both constrained by the conventional model of medicine manufacture.
Traditional manufacturing poses particular problems to the routine practice of personalised medicine. This is of note, given that personalised medicine is expected to transform healthcare by tailoring treatments to single patients or small groups. But designing medicines based on an individual’s specific molecular, physiological and environmental characteristics requires a complex manufacturing process. The numerous steps required to produce CAR-T cells - a personalised therapy revolutionising cancer treatment – exemplifies this. A patient’s own cells must be collected, genetically modified and multiplied. Until now, regulators viewed the bedside administration as the point of ‘product release’. Consequently, the regulators considered each person’s treatment to be a batch for one person – rendering the scalability of personalised medicines infeasible.
Many new medicines therefore need a new manufacturing model, which brings production closer to the patient.
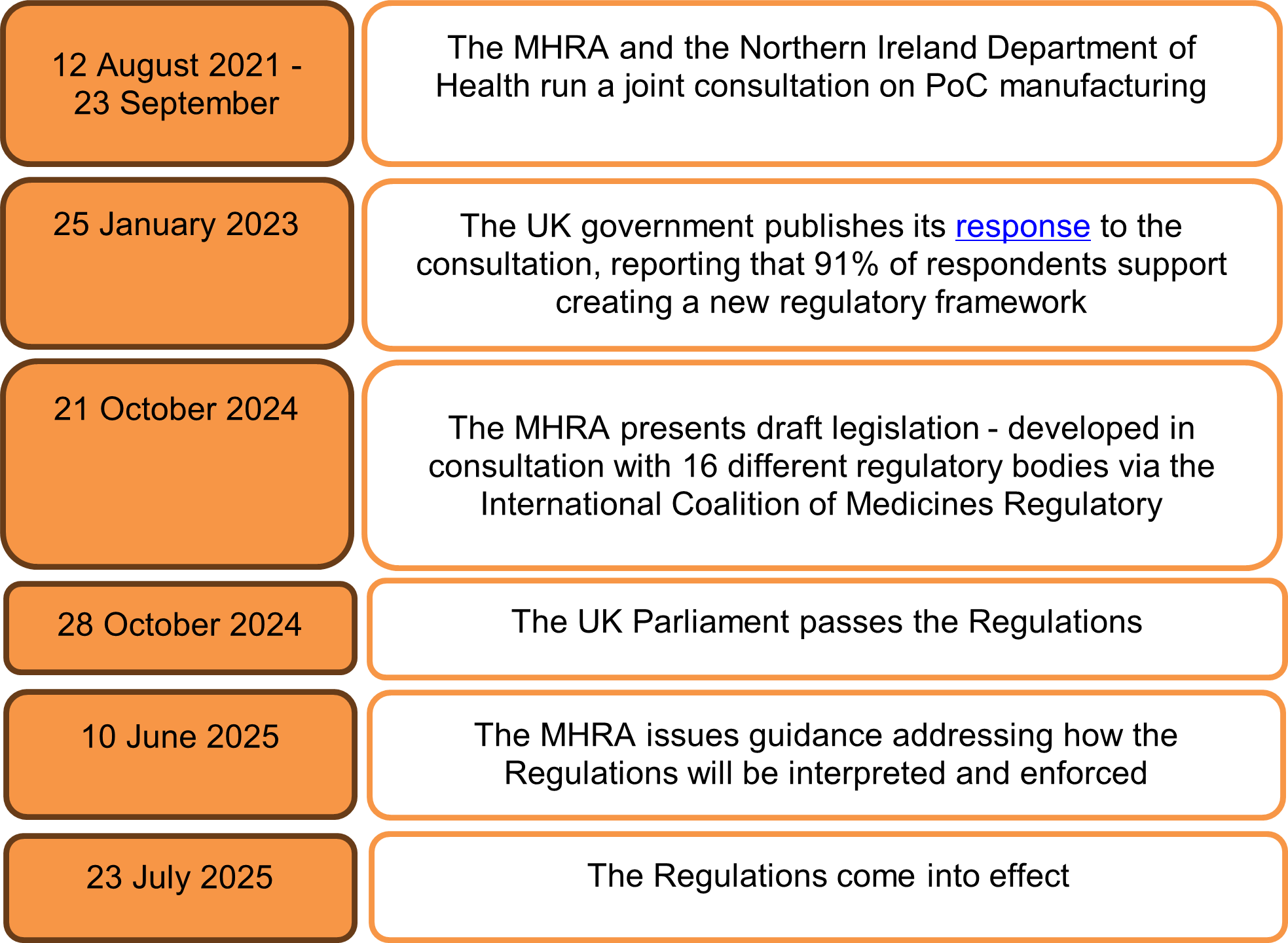
How were the Regulations developed?
The UK now has first-of-its-kind legislation formally addressing the need for a new manufacturing model. The MHRA has been considering such a framework since 2021, when it ran a public consultation on manufacturing medicines nearer to the point of care. Following strong support in the consultation, the Regulations were passed on 10 June 2025 and came into effect on 23 July 2025.
The development of the Regulations – from initial consultation to implementation - is set out below:
What are the changes to medical manufacturing?
The MHRA uses the term ‘decentralised manufacturing’ to describe medical manufacturing outside of central specialised manufacturing facilities. The Regulations enable decentralised manufacturing by introducing two new licences, for PoC and modular manufacturing respectively. Applicants for both new licence types are expected to be pharmaceutical companies, academic institutions, or NHS-affiliated research organisations seeking to use decentralised manufacturing to bring therapies to the UK market.
PoC manufacturing licences enable medicines to be developed directly at the patient’s bedside (whether that be in a hospital or the home) or in very close proximity (such as a hospital). The intention is to make CAR-T cell therapies in days rather than months, and to allow the use of gene therapies with minutes of shelf life. PoC manufacturing also raises exciting possibilities to 3D print pharmaceuticals, perhaps in combination with AI, to produce medicines in real-time.
Modular manufacturing licences have separate use cases. This licence allows the production of medicines using self-contained, relocatable units so that medicine manufacture can be adapted depending on the local need. For example, modular manufacturing would provide flexibility to rapidly scale up the delivery of vaccines during a pandemic. Bringing portable manufacturing units directly to patients also offers the safer treatment of individuals who are immunocompromised or too unwell to travel.
Under the Regulations, the holders of both PoC manufacturing licences and modular manufacturing licences are the central ‘hub’ in a new hub-and-spoke framework. To scale up production, the licence holder manufactures certain parts of the medicine. For example, the non-personalised components of a gene therapy (such as the gene in the vector) would be made at a central, specialised factory. To scale out, sites such as hospitals act as ‘spokes’ and complete the manufacturing process under the licence holder’s supervision. As the MHRA will only oversee compliance through the control sites, product release occurs at the licence holder’s central manufacturing facility. Crucially, this moves product release away from the bedside.
How do the Regulations affect industry members?
Bringing the final manufacturing step closer to patients presents exciting opportunities for the development of innovative medicines. Whilst the Regulations do have safeguarding protocols, industry members may be concerned given the significant shift from the traditional manufacturing model.
In particular, ensuring Good Manufacturing Practices at all spoke sites poses challenges for licence holders. Is it realistic that a licence holder can rigorously monitor quality, dosage and contamination across so many spoke sites? Any failures to effectively supervise spoke sites could increase the numbers of adverse events and, ultimately, product recalls.
The challenge faced by licence holders is compounded by the fact that recalling products is more logistically complex under a decentralised framework. Conducting a recall from so many disparate spoke sites, instead of a few central locations, may be expensive and affect insurance coverage. However, difficulties with product recalls are inherent to any model for the superfast delivery of treatments to patients. This is because, once a defect is identified, there may not be enough time to warn healthcare providers before the impacted doses are given to more patients.
Pharmaceutical companies must adapt to the Regulations, and a future where healthcare delivery gets faster, by developing mitigation strategies. For example, licence holders may implement rapid-alert procedures for ultra-short shelf-life products and use automated digital notifications between modular sites.
What is next?
The UK Government hopes that the Regulations will foster the safe development of innovative medicines and, ultimately, stimulate growth in the life sciences sector. The UK’s Minister for Secondary Care, Karin Smyth, said the framework was introduced “pre-emptively”, before the widespread adoption of innovative medicines. This suggests a real shift in the legislative approach, with policymakers attempting actively to anticipate medical advances rather than to react to them.
The world-first framework will be monitored closely by international regulators. A comparable framework is under development in the USA, where the term ‘distributed manufacturing’ is used instead of ‘decentralised manufacturing’. In 2023, the Center for Biologics Evaluation and Research said that distributed manufacturing poses significant regulatory challenges, such as comparability between sites, adequate staff training and maintaining aseptic environments. Successful implementation of the Regulations in the UK will perhaps assuage these concerns.
It is now important that international models on decentralised manufacturing align, given the global nature of pharmaceutical trade and supply chains. In the USA, the pharmaceutical trade body, PhRMA, has explicitly encouraged the FDA to harmonise its approach with other authorities. Pharmaceutical companies should therefore pay close attention to the UK Regulations and anticipate the introduction of potentially similar models for medicine manufacturing elsewhere.

/Passle/65b10a249576f7f0a5a2f163/MediaLibrary/Images/2026-02-13-09-54-00-479-698ef4b8fb33fd3b3497e8d8.png)
/Passle/65b10a249576f7f0a5a2f163/MediaLibrary/Images/2026-02-09-11-01-29-833-6989be89a15d6a86d5d4e081.jpg)
/Passle/65b10a249576f7f0a5a2f163/MediaLibrary/Images/2026-01-30-11-19-05-331-697c93a9151bcc6789224659.png)
/Passle/MediaLibrary/Images/2026-01-14-16-14-00-251-6967c0c85969610affcf3f3a.jpg)